Data-Driven Discovery and Design of New Superelastic Ceramics
Superelastic ceramics are an emerging class of materials that offer promise for significant energy dissipation in Army blast/impact protection applications. Current superelastic ceramics with promising dissipative properties are essentially limited to a single material, zirconia. Recent work in the Schuh and Radovitzky research groups funded by the Institute for Soldier Nanotechnologies has thoroughly investigated zirconia superelastic ceramics, and achieved several key demonstrations, including scale-up to cm-scale specimens in two specific product forms (single crystals and granular packings). There is, however, one even more desirable full-scale product form that would be of even greater technological interest, namely, bulk polycrystalline superelastic ceramics. Work on zirconia has revealed the key physics of this class of materials, and researchers are now able to predict a priori the structures of ceramics that can achieve the goal of a polycrystalline superelastic condition. Specifically, new superelastic ceramics must have carefully tailored crystalline lattices with improved crystallographic compatibility between the superelastic phases. This compatibility will allow the material to avoid cracking and thus maximize energy dissipation and allow for repeatable energy absorption events. This project will use the modern toolkits of materials discovery and design to identify promising new compositions, which will then be reduced to practice and validated.
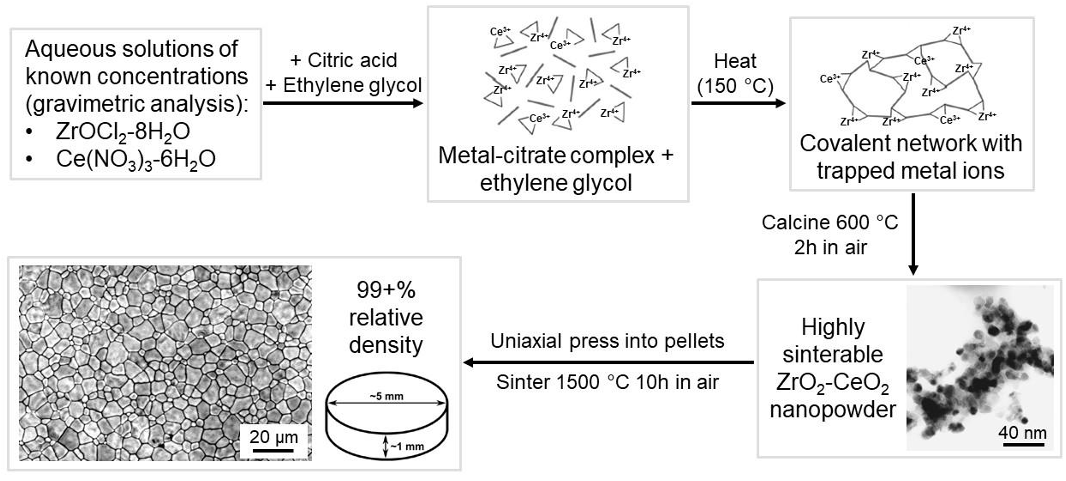
Example illustrating a flowchart of a modified Pechini method for synthesizing any oxide as needed for the research program.
Gravimetric analysis for precise cation content; citric acid to chelate cations & prevent precipitation; ethylene glycol and heat to polymerize system; burn-off organics to get dry powder; press & sinter.

